OsteoDx Invests $2M to Accelerate Commercialization
OsteoDx Invests $2M to Accelerate Commercialization
Research Studies Indicate Improved Diagnostic for Assessing Skeletal Health
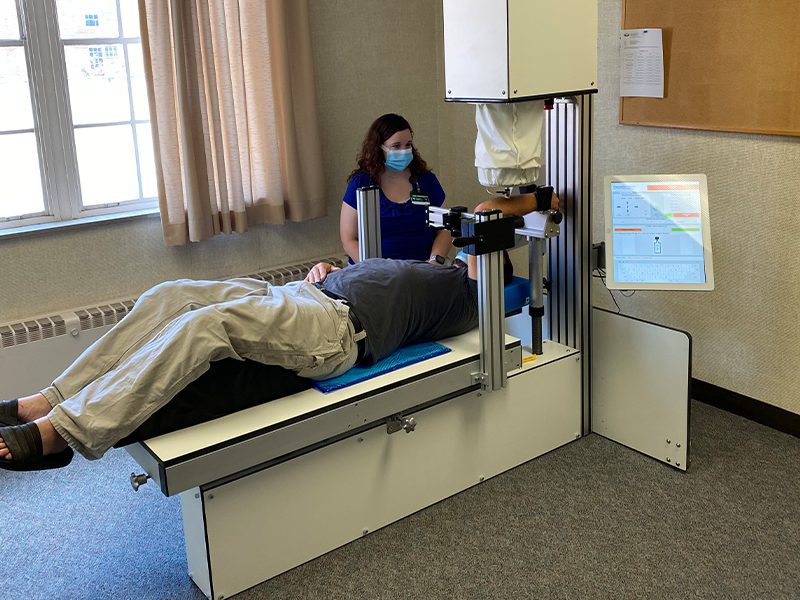
Athens, OH-based OsteoDx, Inc. hopes to better assess bone strength. Along with additional TechGrowth Fund investment, the company has submitted for a Phase IIB SBIR Grant and received favorable initial scoring to accelerate commercialization of its proprietary Cortical Bone Mechanics Technology™ (CBMT). Studies demonstrate that CBMT™ delivers accurate, non-invasive measurement of ulna bone strength that is not currently available from other diagnostics. This funding will position OsteoDx to apply for FDA approval for use of the CBMT™ device to non-invasively estimate bone strength. Commercialization of CBMT could significantly reduce the $50B+ of annual US healthcare costs attributed to osteoporosis alone.
“This funding positions OsteoDx to achieve our next big milestone,” said Ron Lachey, acting CEO and Executive in Residence for TechGrowth Ohio. “We are laser-focused on achieving FDA approval because the market needs this technology.”
OsteoDx’s CBMT technology was developed to enhance the diagnosis of bone health diseases, such as osteoporosis. OsteoDx is currently conducting The STRONGER Study at 5 sites that are testing subjects utilizing CBMT devices.
“Our research indicates that we can non-invasively and painlessly deliver an accurate measurement that is almost directly correlated with actual whole bone strength,” said Brian Clark, Ph.D., co-founder of OsteoDx. “No other technology actually makes that claim.”
Researchers at OsteoDx also established that CBMT is sensitive to detecting change in bone strength and provides information about cortical bone that is unique and independent of Bone Mineral Density (BMD), the current benchmark assessment of osteoporosis, which suggests CBMT may yield additional and clinically significant information about bone health. Osteoporosis is a common medical condition causing progressive weakening of bones, eventually leading to non-traumatic or fragility fractures.
“The development of the CBMT device has been challenging, but we are fortunate to work with some brilliant researchers to develop and refine a novel, user-friendly, and effective system that accurately measures ulna cortical bone strength,” said Andrew Dick, OsteoDx Director of Engineering, and one of the inventors of the CBMT device.
About OsteoDx
Cortical Bone Mechanics Technology was developed by world-renowned experts in bone mechanics at Ohio University in Athens, Ohio.
OsteoDx, Inc. was originally founded in September 2016 as AEIOU Scientific, an Ohio-based LLC. Working with TechGrowth Ohio, the company converted to OsteoDx, Inc., a Delaware C-Corporation, in 2020. The leadership team is comprised of acknowledged experts in bone health/fracture research, mechanical engineering, vibration analysis, and commercial startup experience with successful corporate exits. OsteoDx has also been selected and featured as one of 20 start-ups nationwide by the Association of Public and Land-grant Universities and the Association of American Universities for the 2019 University Innovation & Entrepreneurship Showcase in Washington, D.C. Moreover, in 2020, they were selected as the “Most Innovative Technology” by NASA iTech Ohio and were a finalist for this award at the national level. You can learn more about OsteoDx at www.osteodx.com.